Aciclovir (INN, BAN) /eɪˈsaɪklɵvɪər/ or acyclovir (USAN, former BAN), is a guanosine analogue antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox and shingles. Other uses include: prevention cytomegalovirus infections following transplant and infections due to Epstein-Barr virus.
Potentially serious side effects include kidney dysfunction and low platelets. Common side effects include nausea and diarrhea. Greater care is recommended in those with poor liver or kidney function. It is generally considered safe for use in pregnancy with no harms having been observed.
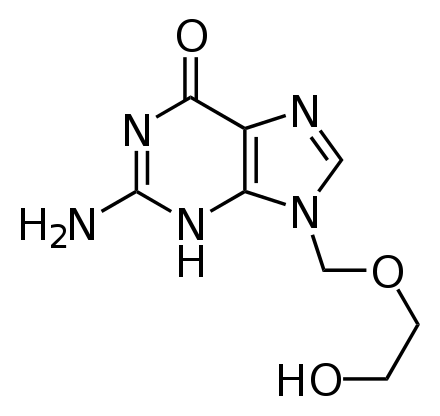
It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system. Brand names include: Cyclovir and Zovirax among others. The chemical name is acycloguanosine (ACV).
Medical use
methyl)-1H-purin-6(9H)-one_200.svg/120px-2-amino-9-((2-hydroxyethoxy)methyl)-1H-purin-6(9H)-one_200.svg.png)
Aciclovir is used for the treatment of herpes simplex virus and varicella zoster virus infections, including:
- Genital herpes simplex (treatment and prevention)
- Herpes simplex labialis (cold sores)
- Shingles
- Acute chickenpox in immunocompromised patients
- Herpes simplex encephalitis
- Acute mucocutaneous HSV infections in immunocompromised patients
- Herpes of the eye and herpes simplex blepharitis (a chronic (long-term) form of herpes eye infection)
- Prevention of herpes viruses in immunocompromised people (such as people undergoing cancer chemotherapy)
Oral aciclovir, however, does not appear to decrease the risk of pain after shingles. In those with herpes of the eye, aciclovir was found to be more effective than idoxuridine or vidarabine in relative number of successfully healed eyes.
Intravenous aciclovir is effective to treat severe medical conditions caused by different species of herpes virus family includes severe localized infections of herpes virus, severe genital herpes, chickenpox & encephalitis. It is also effective in systemic or traumatic herpes infections, eczema herpeticum and Herpes simplex meningitis. Reviews of research dating from the 1980s show there is some effect in reducing the number and duration of lesions if aciclovir is applied at an early stage of an outbreak. Recent research shows effectiveness of topical Aciclovir in both the early and late stages of the outbreak as well as improving methodologically and in terms of statistical certainty from previous studies. Aciclovir trials show that this agent has no role in preventing HIV transmission, but it can help slow HIV disease progression in people not taking anti-retroviral therapy (ART). This finding emphasizes the importance of testing simple, inexpensive non-ART strategies, such as aciclovir and cotrimoxazole, in people with HIV.
Pregnancy
Classified as a Category B Drug, the CDC and others have declared that during severe recurrent or first episodes of genital herpes, aciclovir may be used. For severe HSV infections (especially disseminated HSV), IV aciclovir may also be used. Studies in mice, rabbits and rats (with doses more than 10 times the equivalent of that used in humans) given during organogenesis have failed to demonstrate birth defects. Studies in rats in which they were given the equivalent to 63 times the standard steady-state humans concentrations of the drug on day 10 of gestation showed head and tail anomalies.
Aciclovir is recommended by the CDC for treatment of Varicella during pregnancy, especially during the second and third trimesters
Aciclovir is excreted in the breast milk, therefore it is recommended that caution should be used in breast-feeding women. It has been shown in limited studies that the nursing infant is exposed to approximately 0.3Â mg/kg/day following oral administration of aciclovir to the mother. If nursing mothers have herpetic lesions near or on the breast, breast-feeding should be avoided.
Adverse effects
Systemic therapy
Common adverse drug reactions (≥1% of patients) associated with systemic aciclovir therapy (oral or IV) include: nausea, vomiting, diarrhea, encephalopathy (with IV use only), injection site reactions (with IV use only) and headache. In high doses, hallucinations have been reported. Infrequent adverse effects (0.1â€"1% of patients) include: agitation, vertigo, confusion, dizziness, oedema, arthralgia, sore throat, constipation, abdominal pain, hair loss, rash and weakness. Rare adverse effects (<0.1% of patients) include: coma, seizures, neutropenia, leukopenia, crystalluria, anorexia, fatigue, hepatitis, Stevensâ€"Johnson syndrome, toxic epidermal necrolysis, thrombotic thrombocytopenic purpura and anaphylaxis.
Intravenous aciclovir may cause reversible nephrotoxicity in up to 5% to 10% of patients because of precipitation of aciclovir crystals in the kidney. Aciclovir crystalline nephropathy is more common when aciclovir is given as a rapid infusion and in patients with dehydration and preexisting renal impairment. Adequate hydration, a slower rate of infusion, and dosing based on renal function may reduce this risk.
Topical therapy
Aciclovir topical cream is commonly associated (≥1% of patients) with: dry or flaking skin or transient stinging/burning sensations. Infrequent adverse effects include erythema or itch. When applied to the eye, aciclovir is commonly associated (≥1% of patients) with transient mild stinging. Infrequently (0.1â€"1% of patients), ophthalmic aciclovir is associated with superficial punctate keratitis or allergic reactions.
Drug interactions

Ketoconazole: In-vitro replication studies have found a synergistic, dose-dependent antiviral activity against HSV-1 and HSV-2 when given with aciclovir. However, this effect is not been clinically established and more studies need to be done to evaluate the true potential of this synergy.
Probenecid: Reports of increased half life of aciclovir, as well as decreased urinary excretion and renal clearance have been shown in studies where probenecid is given simultaneously with aciclovir.
Interferon: Synergistic effects when administered with aciclovir and caution should be taken when administering aciclovir to patients recieiving IV interferon.
Zidovudine: Although administered often with aciclovir in HIV patients, neurotoxicity has been reported in at least one patient who presented with extreme drowsiness and lethargy 30â€"60 days after receiving IV aciclovir; symptoms resolved when aciclovir was discontinued.
Detection in biological fluids
Aciclovir may be quantitated in plasma or serum to monitor for drug accumulation in patients with renal dysfunction or to confirm a diagnosis of poisoning in acute overdose victims.
Mechanism of action
Aciclovir is converted by viral thymidine kinase to aciclovir monophosphate, which is then converted by host cell kinases to aciclovir triphosphate (ACV-TP). ACV-TP, in turn, competitively inhibits and inactivates HSV-specified DNA polymerases preventing further viral DNA synthesis without affecting the normal cellular processes.
Microbiology
Aciclovir is active against most species in the herpesvirus family. In descending order of activity:
- Herpes simplex virus type I (HSV-1)
- Herpes simplex virus type II (HSV-2)
- Varicella zoster virus (VZV)
- Epstein-Barr virus (EBV)
- Cytomegalovirus (CMV) â€" least activity
Resistance
Resistance to aciclovir is rare, but is more common in patients on chronic antiviral prophylaxis (transplant recipients, people with acquired immunodeficiency syndrome due to HIV infection). Mechanisms of resistance in HSV include deficient viral thymidine kinase; and mutations to viral thymidine kinase or DNA polymerase, altering substrate sensitivity.
Pharmacokinetics
Acyclovir is poorly water soluble and has poor oral bioavailability (15â€"30%), hence intravenous administration is necessary if high concentrations are required. When orally administered, peak plasma concentration occurs after 1â€"2 hours. Aciclovir has a high distribution rate; protein binding is reported to range from 9 to 33%. The elimination half-life (t1/2) of aciclovir depends according to age group; neonates have a t1/2 of 4 hours, children 1â€"12 years have a t1/2 of 2â€"3 hours whereas adults have a t1/2 of 3 hours.
History
Aciclovir was seen as the start of a new era in antiviral therapy, as it is extremely selective and low in cytotoxicity.Since discovery in mid 1970s, it is being used as an effective drug for the treatment of infections caused by most known species of the Herpes virus family including Herpes zoster & Varicella zoster viruses. Nucleosides isolated from a Caribbean sponge, Cryptotethya crypta, were the basis for the synthesis of aciclovir. It was codiscovered by Howard Schaffer following his work with Robert Vince, S. Bittner and S. Gurwara on the adenosine analog acycloadenosine which showed promising antiviral activity. Later, Schaffer joined Burroughs Wellcome and continued the development of aciclovir with pharmacologist Gertrude B. Elion. A U.S. patent on aciclovir listing Schaffer as inventor was issued in 1979.
Vince later went on to invent abacavir, an nRTI drug for HIV patients. Elion was awarded the 1988 Nobel Prize in Medicine, partly for the development of aciclovir. Richard Whitley, a University of Alabama at Birmingham researcher and pioneer in antiviral therapy, was the first to successfully use the drug in humans.
Brand names
Brand names include: Cyclovir, Herpex, Acivir, Acivirax, Zovirax, Zoral, Xovir and Imavir
See also
- Valaciclovir
- Idoxuridine
Notes
References
Further reading
- Hazra, S; Konrad, M; Lavie, A (2010). "The sugar ring of the nucleoside is required for productive substrate positioning in the active site of human deoxycytidine kinase (dCK): Implications for the development of dCK-activated acyclic guanine analogues". Journal of Medicinal Chemistry 53 (15): 5792â€"800. doi:10.1021/jm1005379. PMC 2936711. PMID 20684612.Â
- Harvey Stewart C. in Remington’s Pharmaceutical Sciences 18th edition: (ed. Gennard, Alfonso R.) Mack Publishing Company, 1990. ISBN 0-912734-04-3.
- Huovinen P., Valtonen V. in Kliininen Farmakologia (ed. Neuvonen et al.). Kandidaattikustannus Oy, 1994. ISBN 951-8951-09-8.
- Périgaud C., Gosselin G., Imbach J. -L.: Nucleoside analogues as chemotherapeutic agents: a review. Nucleosides and nucleotides 1992; 11(2â€"4)
- Rang H.P., Dale M.M., Ritter J.M.: Pharmacology, 3rd edition. Pearson Professional Ltd, 1995. 2003 (5th) edition ISBN 0-443-07145-4; 2001 (4th) edition ISBN 0-443-06574-8; 1990 edition ISBN 0-443-03407-9.
External links
- U.S. National Library of Medicine: Drug Information Portalâ€"Aciclovir



0 komentar :
Posting Komentar