Rifaximin (Rifagut) is a semisynthetic antibiotic based on rifamycin. It has poor oral bioavailability, meaning that very little of the drug will be absorbed into the blood stream when it is taken orally. Rifaximin is used in the treatment of traveler's diarrhea and hepatic encephalopathy, for which it received orphan drug status from the U.S. Food and Drug Administration in 1998.
Uses
Rifaximin is licensed by the U.S. Food and Drug Administration to treat traveler's diarrhea caused by E. coli. Clinical trials have shown that rifaximin is highly effective at preventing and treating traveler's diarrhea among travelers to Mexico, with few side effects and low risk of developing antibiotic resistance. It is not effective against Campylobacter jejuni, and there is no evidence of efficacy against Shigella or Salmonella species.
It may be efficacious in relieving chronic functional symptoms of bloating and flatulence that are common in irritable bowel syndrome (IBS).
There was recently a pilot-study done on the efficacy of rifaximin as a means of treatment for rosacea, according to the study, induced by the co-presence of small intestinal bacterial overgrowth.
In the United States, rifaximin has orphan drug status for the treatment of hepatic encephalopathy. Although high-quality evidence is still lacking, rifaximin appears to be as effective as or more effective than other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster. The drawbacks to rifaximin are increased cost and lack of robust clinical trials for HE without combination lactulose therapy.
Mechanism of action

Rifaximin interferes with transcription by binding to the β-subunit of bacterial RNA polymerase. This results in the blockage of the translocation step that normally follows the formation of the first phosphodiester bond, which occurs in the transcription process.
Efficacy
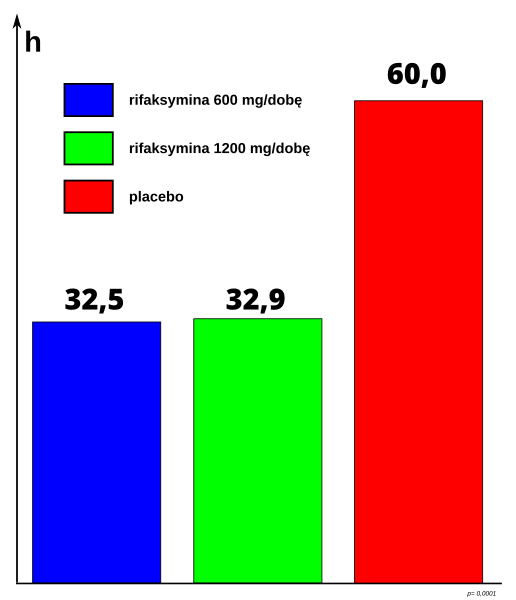
A 2011 study in patients with IBS (sans constipation) indicated 11% showed benefits over a placebo. The study was supported by Salix Pharmaceuticals, the patent holder. A 2010 study in patients treated for Hepatic Cirrhosis with hospitalization involving Hepatic encephalopathy resulted in 22% of the Rifaxmin treated group experiencing a breakthrough episode of Hepatic encephalopathy as compared to 46% of the placebo group. The majority patients were also receiving Lactulose therapy for prevention of hepatic encephalopathy in addition to Rifaximin.
Availability
In the United States, Salix Pharmaceuticals holds a US Patent for rifaximin and markets the drug under the name Xifaxan, available in tablets of 200 mg and 550 mg. While FDA approved for traveler’s diarrhea and (marketing approved for) hepatic encephalopathy, Xifaxan has not yet received FDA approval for IBS. Medical insurance may not cover drug costs when prescribed for “off-label†uses, such as Xifaxan prescribed for IBS. No generic formulation is available in the US and none will appear while the FDA approval process is ongoing. If Xifaxan receives full FDA approval for hepatic encephalopathy it is likely that Salix will maintain marketing exclusivity and be protected from generic formulations until March 24, 2017. Price quotes received on February 21, 2013 for Xifaxan 550 mg in the Denver Metro area were between $23.57 and $26.72 per tablet.
Rifaximin is approved in 33 countries for GI disorders,. On August 13, 2013, Health Canada issued a Notice of Compliance to Salix Pharmaceuticals Inc. for the drug product Zaxine. Though lower cost, generic formulations of rifaximin may be offered via Canadian web domains, these products have not been evaluated or authorized for sale in Canada and may not be in compliance with Canadian law. The Canadian government, via Health Canada, has warned of the dangers of purchasing drugs over the internet. The US government, through the FDA, and the National Association of Boards of Pharmacy are among other organizations that have issued similar warnings about “Canadian†and other drugs available online and offer resources for consumers to help ensure the safety of internet drugs. In India it is available under the brand name of CIBOZ, XIFAPILL 200/400 (ORDAIN EXELTIS).
References
External links
- FDA label approved for Xifaxan (PDF warning)
- Xifaxan (manufacturer's website)




BalasHapusThanks a lot for sharing the details about Rifaximin and such medical details.
check at Buy Rifagut tablets online
with this also check similar and equivalent medicines at Online Generic Medicine